25 ++ letermovir 140612-Letermovir dosis
Letermovir comes as a tablet to take by mouth It is usually taken with or without food once a day Your doctor will probably tell you to start taking letermovir after your receive the transplant and to stop taking the medication 100 days after the transplant Take letermovir at around the same time every dayThe clinical relevance is unknown Letermovir inhibited efflux transporters Pgp, breast cancer resistance protein (BCRP), bile salt export pump (BSEP), multidrug resistanceassociated protein 2 (MRP2), OAT3, and hepatic uptake transporter OATP1B1/3 in vitroFind Ambeed, IncAMBH93D52F8A MSDS, related peerreviewed papers, technical documents, similar products & more at SigmaAldrich

Pdf Letermovir And Inhibitors Of The Terminase Complex A Promising New Class Of Investigational Antiviral Drugs Against Human Cytomegalovirus
Letermovir dosis
Letermovir dosis-A procedure that replaces diseased bone marrow with healthy bone marrow) and are at increased risk of developing a CMV infection Letermovir is in a class of medications called antiviralsLetermovir is a new agent approved for CMV prophylaxis in hematopoietic stem cell transplantation and is associated with less toxicity This study aims to assess the effectiveness and safety of letermovir in solid organ transplantation Methods A singlecenter, matched cohort study was performed on 31 transplant recipients who were converted



Full Text Letermovir And Inhibitors Of The Terminase Complex A Promising New Cl Idr
Uses for Letermovir Prevention of CMV Infection and Disease Prophylaxis to prevent CMV infection and disease in adult CMVseropositive recipients (R ) of an allogeneic hematopoietic stem cell transplant (HSCT) 1 3 Designated an orphan drug by FDA for prevention of CMV viremia and disease in atrisk populations 2Letermovir/Cyclosporine Interactions This information is generalized and not intended as specific medical advice Consult your healthcare professional before taking or discontinuing any drug orLetermovir (AIC246, MK28) is a novel antiCMV compound which targets the viral terminase complex and remains active against virus resistant to DNA polymerase inhibitors Selleck's Letermovir (AIC246) has been cited by 1 publication bioRxiv, , Purity & Quality Control
Don't delay your care at Mayo Clinic Schedule your appointment now for safe inperson care Learn more Mayo Clinic facts about coronavirus disease 19 (COVID19) Our COVID19 patient and visitor guidelines, plus trusted health information Latest on COVID19 vaccination by site Arizona patient vaccination updates Arizona, Florida patient vaccination updates Florida, Rochester patientLetermovir, compared with placebo, reduced the incidence of CMV reactivation and disease in CMVseropositive adults undergoing allogeneic HSCT This SMC advice takes account of the benefits of a Patient Access Scheme (PAS) that improves the costeffectiveness of letermovirLetermovir is an antiviral drug It works by stopping the growth of the virus How to use Letermovir 240 Mg/12 Ml Intravenous Solution Read the Patient Information Leaflet if available from your
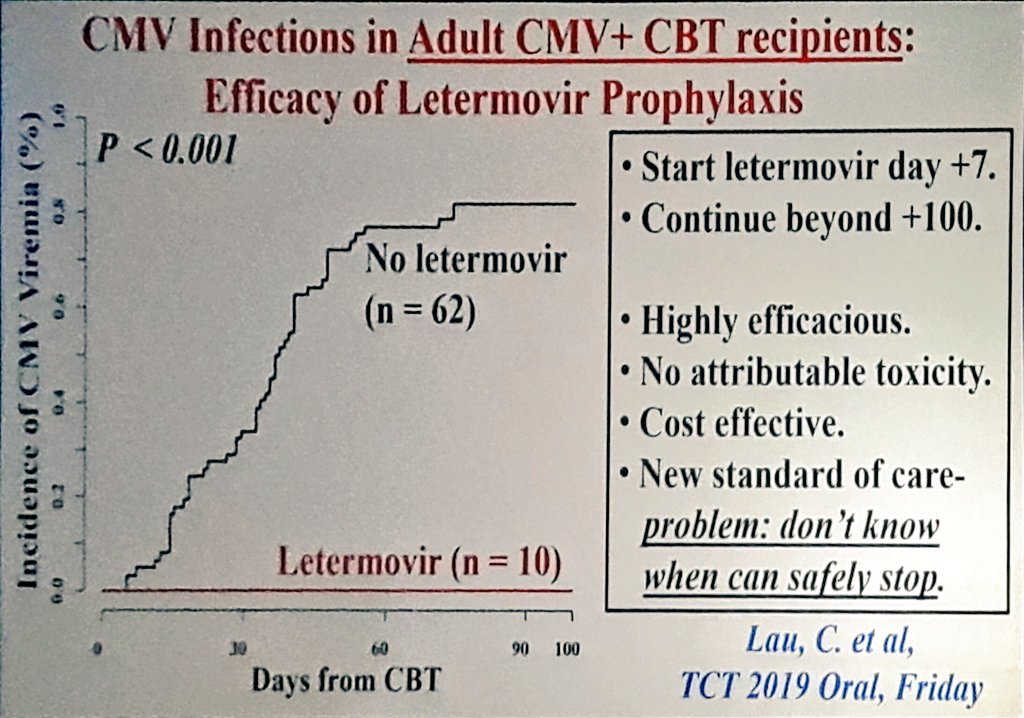
Letermovir prophylaxis through day 100 post transplant is safe and effective compared with alternative CMV prophylaxis strategies following adult cord blood and haploidentical cord blood transplantation Bone Marrow Transplant, 55 (), pp CrossRef View Record in Scopus Google Scholar 27Detect CMV drug resistance in your critical patients with Viracor's CMV drug resistance test for Letermovir, Ganciclovir, Foscarnet and Cidofovir to evaluate UL54, UL56 and UL97 genotypic mutations Viracor's genotypic antiviral sequencing test now includes CMV resistance letermovirLetermovir is an inducer of CYP2B6 in vitro;


Letermovir



Prevymis Letermovir Official Consumer Site
Letermovir// letermovir DMF, CEP, Written Confirmations, FDF, Prices, Patents, Patents & Exclusivities, Dossier, Manufacturer, Licensing, Distributer, Suppliers, NewsLetermovir es una medicina antiviral usada para ayudar a prevenir una infección por citomegalovirus (CMV) después de un trasplante de células madres (medula ósea) de un donante Letermovir es para el uso en los adultos que son seropositivos para CMV Seropositivo significa que el virus está en su sangre aunque noProtocol Description This research study evaluates the drug letermovir (LET or MK28) for prevention of cytomegalovirus (CMV) infection in pediatric patients who have received an allogeneic hematopoietic stem cell transplant (HSCT)


Http Www Io Nihr Ac Uk Wp Content Uploads Migrated Letermovir April 16 Pdf



Letermovir Cytomegalovirus Cmv Drug Molecule Stylized 2d Rendering Royalty Free Cliparts Vectors And Stock Illustration Image
US Food and Drug Administration New Hampshire Avenue Silver Spring, MD 993 18INFOFDA () Contact FDAPREVYMIS™(letermovir) tablets, for oral use PREVYMIS™(letermovir) injection, for intravenous use Initial US Approval 17 INDICATIONS AND USAGE PREVYMIS is a CMV DNA terminase complex inhibitor indicated for prophylaxis of cytomegalovirus (CMV) infection and disease in adultOxidative metabolism is considered to be a minor elimination pathway based on in


Q Tbn And9gcrawbl6uxcjb7zcieikwkvos8 Mhpgay Yvoowx7igrpmt457g1 Usqp Cau
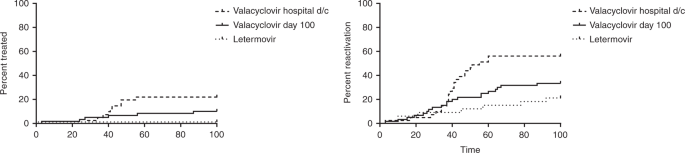


Letermovir Prophylaxis Through Day 100 Post Transplant Is Safe And Effective Compared With Alternative Cmv Prophylaxis Strategies Following Adult Cord Blood And Haploidentical Cord Blood Transplantation Bone Marrow Transplantation
Letermovir (Prevymis®) is accepted for use within NHSScotland Indication under review for prophylaxis of cytomegalovirus (CMV) reactivation and disease in adult CMVseropositive recipients R of an allogeneic haematopoietic stem cell transplant (HSCT)Don't delay your care at Mayo Clinic Schedule your appointment now for safe inperson care Learn more Mayo Clinic facts about coronavirus disease 19 (COVID19) Our COVID19 patient and visitor guidelines, plus trusted health information Latest on COVID19 vaccination by site Arizona patient vaccination updates Arizona, Florida patient vaccination updates Florida, Rochester patientAsk your health care provider or pharmacist if you have questions COMMON BRAND NAME (S) Prevymis


Kegg Drug Letermovir



Menu News Cme Journals All Specialties All Specialties Aesthetics Allergy Immunology Cardiac Vascular Intervention Cardiology Dermatology Endocrinology Gastroenterology Hematology Oncology Hepatology Infectious Disease Nephrology Neurology
Copay Cost Assistance for PREVYMIS™ (letermovir) COPAY COUPON FOR PREVYMIS YOUR ELIGIBLE PRIVATELY INSURED PATIENTS MAY SAVE ON PREVYMIS Eligible patients may visit prevymiscom to learn moreLetermovir use was safe overall, but we found a modestly higher incidence of vomiting and edema in the letermovir group than in the placebo group, a finding that was also seen in the phase 2 trialLetermovir (let er' moe vir) is an antiviral medicine It is used to prevent infections caused by certain kinds of viruses This medicine may be used for other purposes;


2


Bmcinfectdis Biomedcentral Com Track Pdf 10 1186 S 019 4016 1 Pdf
Letermovir is an antiviral medicine used to help prevent cytomegalovirus (CMV) infection after a stem cell (bone marrow) transplant from a donor Letermovir is for use in adults who are seropositive for CMV Seropositive means that the virus is in your blood even if you do not show any symptoms of infection APREVYMIS™ (letermovir) Official Consumer Site If you're an adult who has received an allogeneic bone marrow transplant, taking PREVYMIS can help prevent cytomegalovirus (CMV) infection and disease Pay as little as $15 for PREVYMIS tablets aLetermovir is an antiviral drug It works by stopping the growth of the virus How to use Letermovir 240 Mg/12 Ml Intravenous Solution Read the Patient Information Leaflet if available from your


Letermovir For Cytomegalovirus Prophylaxis Australian Prescriber


Q Tbn And9gcrpfomzt5c8qo4chvjuoy3j3m31olsawffswb2kzsbhmn9ccohc Usqp Cau
Letermovir, a novel CMV viral terminase inhibitor drug, was recently approved for CMV prophylaxis in allogeneic HSCT recipients It has a favorable pharmacokinetic and tolerability profileLetermovir is an antiviral medicine used to help prevent cytomegalovirus (CMV) infection after a stem cell (bone marrow) transplant from a donor Letermovir is for use in adults who are seropositive for CMV Seropositive means that the virus is in your blood even if you do not show any symptoms of infectionLetermovir Memorial Sloan Kettering Cancer Center COVID19 Vaccine Available to MSK Patients As of 3/3, vaccines are available to MSK patients age 18 and over who live in New York State and who were treated for cancer at MSK on or after 1/1/18 For more info, and if you live in NJ Read more



Pharmacology Of Letermovir And Maribavir Download Scientific Diagram



Pdf Letermovir Prophylaxis Is Effective In Preventing Cytomegalovirus Reactivation After Allogeneic Hematopoietic Cell Transplantation Single Center Real World Data
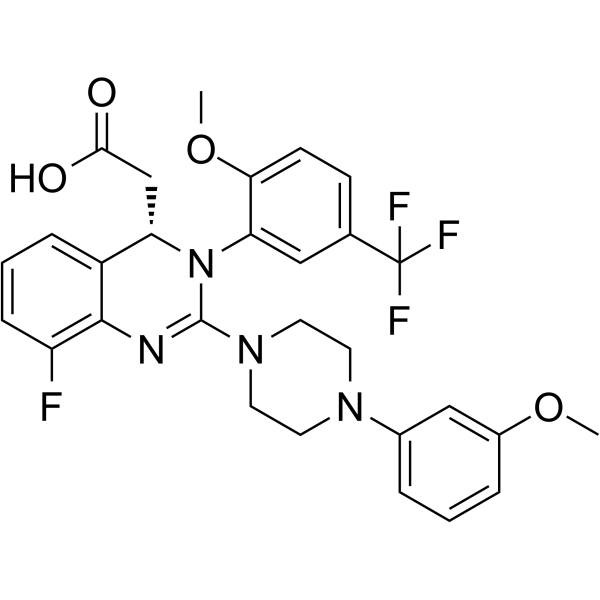
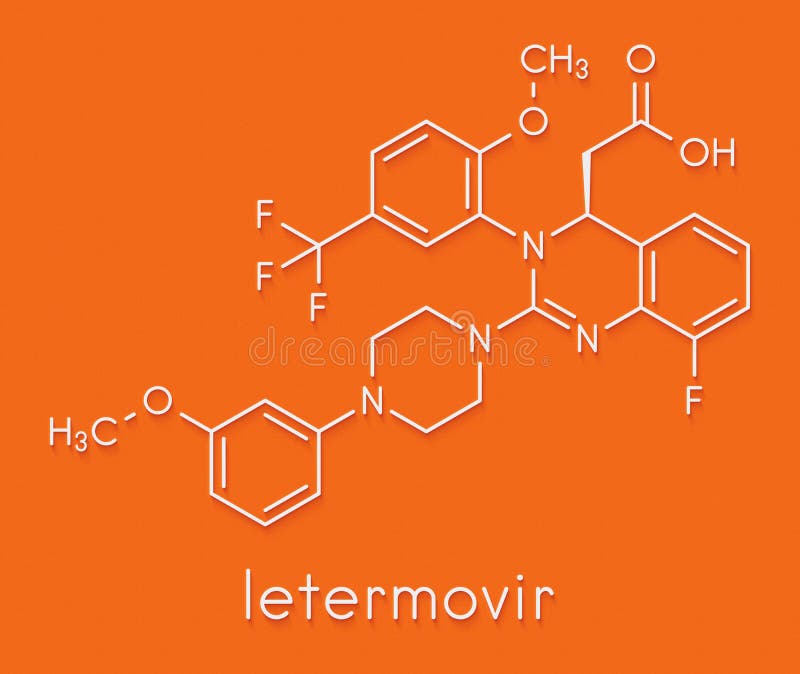
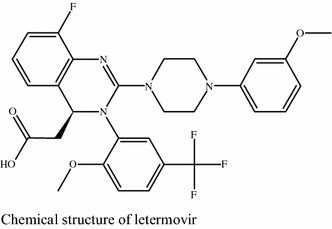
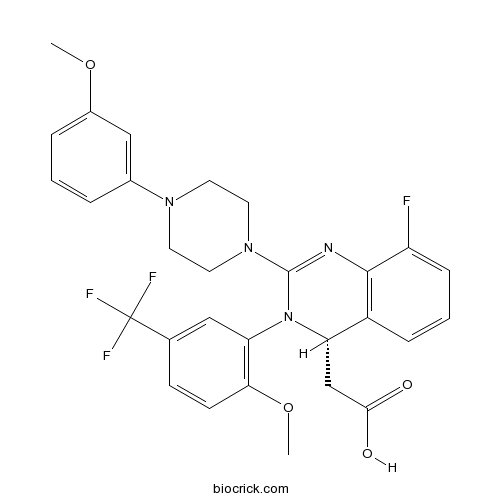
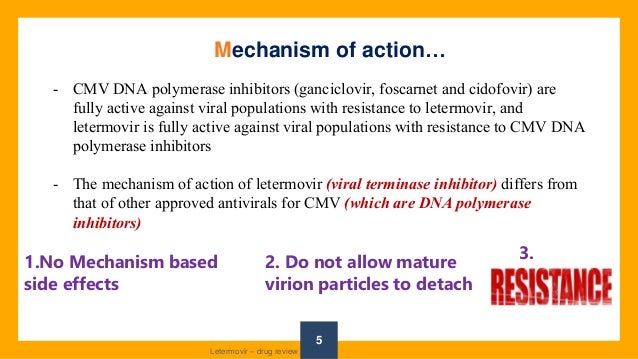
Letermovir is a cytomegalovirus DNA terminase complex inhibitor that interferes with cytomegalovirus genome formation and virion maturationLetermovir (AIC246, MK28) is a novel antiCMV compound which targets the viral terminase complex and remains active against virus resistant to DNA polymerase inhibitors In vitro AIC246 blocks viral replication without inhibiting the synthesis of progeny HCMV DNA or viral proteinsLetermovir is a new agent approved for CMV prophylaxis in hematopoietic stem cell transplantation and is associated with less toxicity This study aims to assess the effectiveness and safety of



Prevymis Letermovir Tablets Uses Dosage Side Effects Interactions Warning



Letermovir For Prophylaxis Of Cytomegalovirus Manifestations In Adult Allogeneic Hematopoietic Stem Cell Transplant Recipients Future Microbiology
Two new CMVactive agents, maribavir and letermovir, have been recently developed for the treatment and prophylaxis of CMV, respectively 2 Both agents have activity against UL97 and UL54mutated CMV Maribavir, although on a fasttrack status, has not yet been approved for the treatment of CMV infection by regulatory agenciesTrade name Prevymis) is an antiviral drug for the treatment of cytomegalovirus (CMV) infections It has been tested in CMV infected patients with allogeneic stem cell transplants and may also be useful for other patients with a compromised immune system such as those with organ transplants or HIV infections The drug was developed by Merck & Co, Inc as investigative compoundLetermovir is one of the most potent antiHCMV agents reported to date, with a median infective dose (ID50) in cell culture reported as 5 nM and a selectivity for lack of cell toxicity of 15,000 From Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases (Eighth Edition), 15



Aicuris Anti Infective Cures Gmbh Aicuris Licensee Merck Msd Received U S Fda Approval Of Prevymis Letermovir For Prevention Of Cytomegalovirus Cmv Infection And Disease In Adult Allogeneic Stem Cell Transplant



Use Of Letermovir For Salvage Therapy For Resistant Cytomegalovirus In A Pediatric Hematopoietic Stem Cell Transplant Recipient
Letermovir (AIC246) is a potent inhibitor of CMV, which targets the viral terminase complex and remains active against virus resistant to DNA polymerase inhibitors For research use only We do not sell to patientsLetermovir es una medicina antiviral usada para ayudar a prevenir una infección por citomegalovirus (CMV) después de un trasplante de células madres (medula ósea) de un donante Letermovir es para el uso en los adultos que son seropositivos para CMVLetermovir is an antiviral medicine used to help prevent cytomegalovirus (CMV) infection after a stem cell (bone marrow) transplant from a donor Letermovir is for use in adults who are seropositive for CMV Seropositive means that the virus is in your blood even if you do not show any symptoms of infection


Letermovir Aic246 Cmv Inhibitor Medchemexpress



Pdf Letermovir And Inhibitors Of The Terminase Complex A Promising New Class Of Investigational Antiviral Drugs Against Human Cytomegalovirus Semantic Scholar
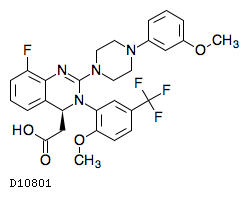
Letermovir is an orally bioavailable, nonnucleoside, 3,4dihydroquinazolinyl acetic acid and inhibitor of the pUL56 subunit of the viral terminase complex of cytomegalovirus (CMV), with potential CMVspecific antiviral activityDon't delay your care at Mayo Clinic Schedule your appointment now for safe inperson care Learn more Mayo Clinic facts about coronavirus disease 19 (COVID19) Our COVID19 patient and visitor guidelines, plus trusted health information Latest on COVID19 vaccination by site Arizona patient vaccination updates Arizona, Florida patient vaccination updates Florida, Rochester patientDetect CMV drug resistance in your critical patients with Viracor's CMV drug resistance test for Letermovir, Ganciclovir, Foscarnet and Cidofovir to evaluate UL54, UL56 and UL97 genotypic mutations Viracor's genotypic antiviral sequencing test now includes CMV resistance letermovir


Letermovir 9173 32 3


Media Empr Com Documents 342 Prevymis Pdf
Letermovir has a bioavailability of 94% in healthy subjects when administered without cyclosporin, 35% in HSCT recipients when administered without cyclosporin, and 85% in HSCT recipients when administered with cyclosporin Label Letermovir's Tmax is 45 min to 225 h Label Time to steady state has been observed to be 910 daysLetermovir (AIC246, MK28) is a novel antiCMV compound which targets the viral terminase complex and remains active against virus resistant to DNA polymerase inhibitors Selleck's Letermovir (AIC246) has been cited by 1 publication bioRxiv, , Purity & Quality ControlThe antiviral drug letermovir is approved for prophylaxis among adult allogeneic HSCT recipients who are cytomegalovirus seropositive through day 100 Although the agent has reduced incidence of



Pdf Letermovir And Inhibitors Of The Terminase Complex A Promising New Class Of Investigational Antiviral Drugs Against Human Cytomegalovirus



Letermovir 9173 32 3 Carbosynth Product
Trade name Prevymis) is an antiviral drug for the treatment of cytomegalovirus (CMV) infections It has been tested in CMV infected patients with allogeneic stem cell transplants and may also be useful for other patients with a compromised immune system such as those with organ transplants or HIV infectionsPREVYMIS™(letermovir) tablets, for oral use PREVYMIS™(letermovir) injection, for intravenous use Initial US Approval 17INDICATIONS AND USAGEPREVYMIS is a CMV DNA terminase complex inhibitor indicated for prophylaxis of cytomegalovirus (CMV) infection and disease in adultLetermovir is indicated for prophylaxis of cytomegalovirus infection and disease in allogeneic hematopoietic stem cell transplant (HSCT) recipients Two‐stage population pharmacokinetic (PK) modeling of letermovir was conducted to support dose rationale and evaluate the impact of intrinsic/extrinsic factors



Clinical Development Of Letermovir And Maribavir Overview Of Human Cytomegalovirus Drug Resistance Sciencedirect



Letermovir For Cmv Prophylaxis In Hsct
Letermovir Dosing Adult Antimicrobial Dosing, Nondialysis Indication CrCl >10 mL/minLetermovir is a substrate of CYP3A (minor), CYP2D6 (minor), UGT1A1, and UGT1A3, and transporters OATP1B1/3 and Pgp;Letermovir (let er' moe vir) is an antiviral medicine It is used to prevent infections caused by certain kinds of viruses The lowest GoodRx price for the most common version of Prevymis is around $6,, 13% off the average retail price of $7,


Letermovir Cytomegalovirus Cmv Drug Molecule Skeletal Formula Stock Illustration Illustration Of Compound Fluorine


Fda Approves Letermovir As Cmv Prophylaxis Mdedge Hematology And Oncology
Letermovir is used to help prevent cytomegalovirus (CMV) infection and disease in certain people who have received a hematopoietic stemcell transplant (HSCT;71 Potential for Other Drugs to Affect PREVYMIS Letermovir is a substrate of organic aniontransporting polypeptide 1B1/3 (OATP1B1/3) and Pglycoprotein (Pgp) transporters and 8 USE IN SPECIFIC POPULATIONS 81 Pregnancy Risk Summary No adequate human data are available to establish whether PREVYMIS poses a risk to pregnancy outcomesPhoompoung et al 4 describe a singlecenter retrospective study of 5 stem cell and organ transplant recipients who were given letermovir for the treatment of resistant or refractory CMV infection, including 3 with asymptomatic CMV viremia, one with CMV syndrome, and another with CMV pneumonitis and colitis


Letermovir First Global Approval Springerlink



Letermovir



Letermovir Aic246 Cas Number 9173 32 3 Cayman Chemical



Letermovir Oral Injection Cigna


Scheme 1 Industrial Applications Of Green Chemistry Status Challenges And Prospects Springerlink


Letermovir Cas 9173 32 3 Novel Anti Cmv Compound High Purity Manufacturer Biocrick



Clinical Real World Experience With Letermovir For Prevention Of Cytomegalovirus Infection In Allogeneic Hematopoietic Cell Transplant Recipients Biology Of Blood And Marrow Transplantation



Letermovir Cytomegalovirus Drug Molecular Model License Download Or Print For 31 45 Photos Picfair


Letermovir Cytomegalovirus Cmv Drug Molecule Stock Illustration Illustration Of Pharmacology Virus



Letermovir


Letermovir Drug Review



Patient Assistance For Prevymis Letermovir



Letermovir Drug Review
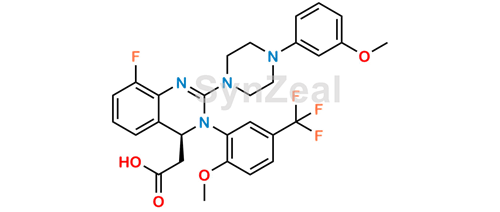


Letermovir 9173 32 3 Synzeal



Prevymis Cost Buy Letermovir Price In India For Chd Treatment Online Available Fda Approved



Letermovir For Cytomegalovirus Prophylaxis In Hematopoietic Cell Transplantation Nejm



Letermovir Cas No 9173 32 3 Simson Pharma Limited



Dosing For Prevymis Letermovir



Prevymis Dosage Rx Info Uses Side Effects



Letermovir Livertox Ncbi Bookshelf



A Single Center Experience Of Letermovir For The Prevention Of Cmv Infection In Cmv Seropositive Allogeneic Cell Transplant Allo Hct Recipients Biology Of Blood And Marrow Transplantation


Letermovir C29h28f4n4o4 Chemspider


Media Empr Com Documents 342 Prevymis Pdf



Index
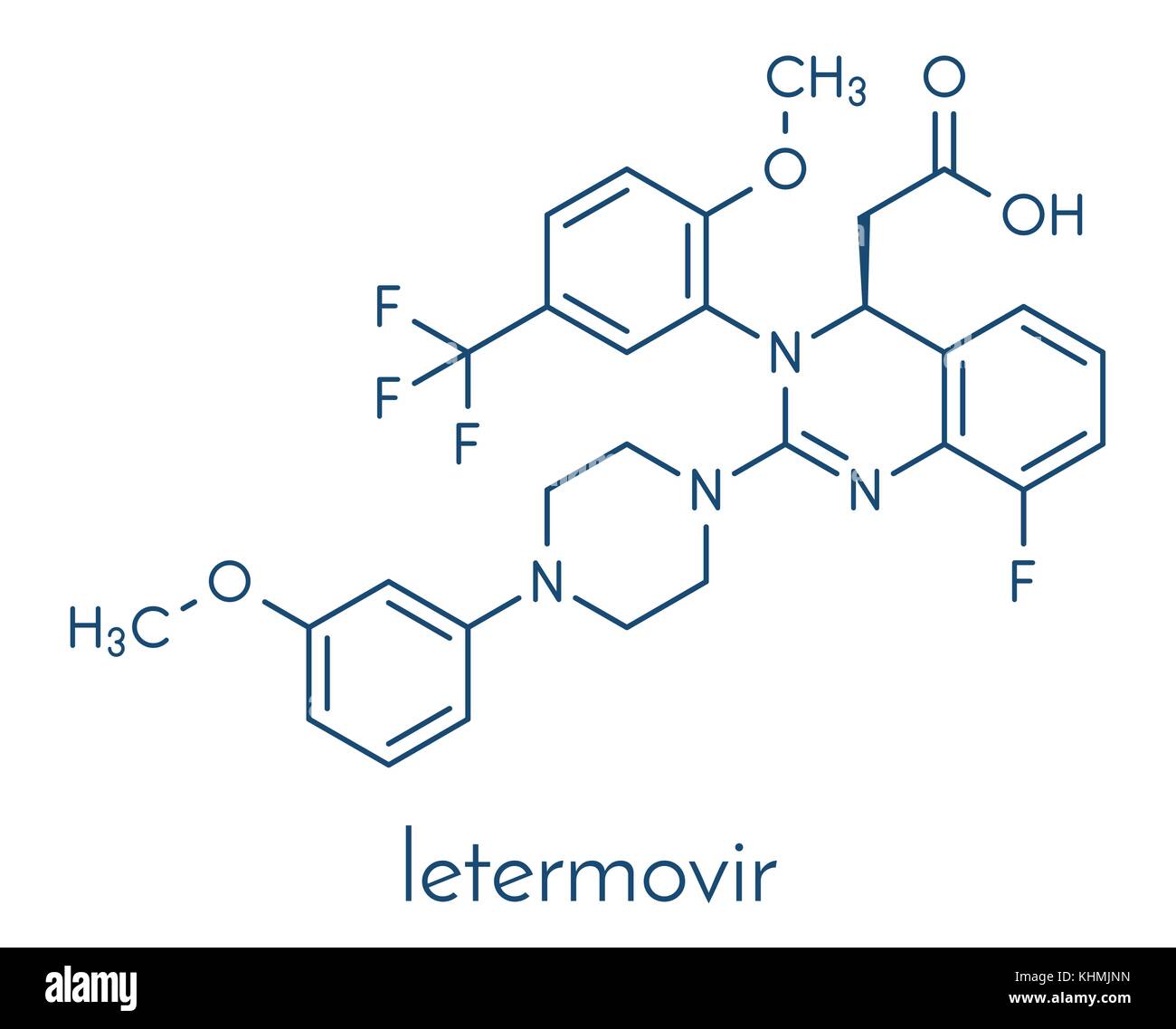


Letermovir Cytomegalovirus Cmv Drug Molecule Skeletal Formula Stock Vector Image Art Alamy



Pdf Letermovir For The Prevention Of Cytomegalovirus Infection And Disease In Transplant Recipients An Evidence Based Review Semantic Scholar
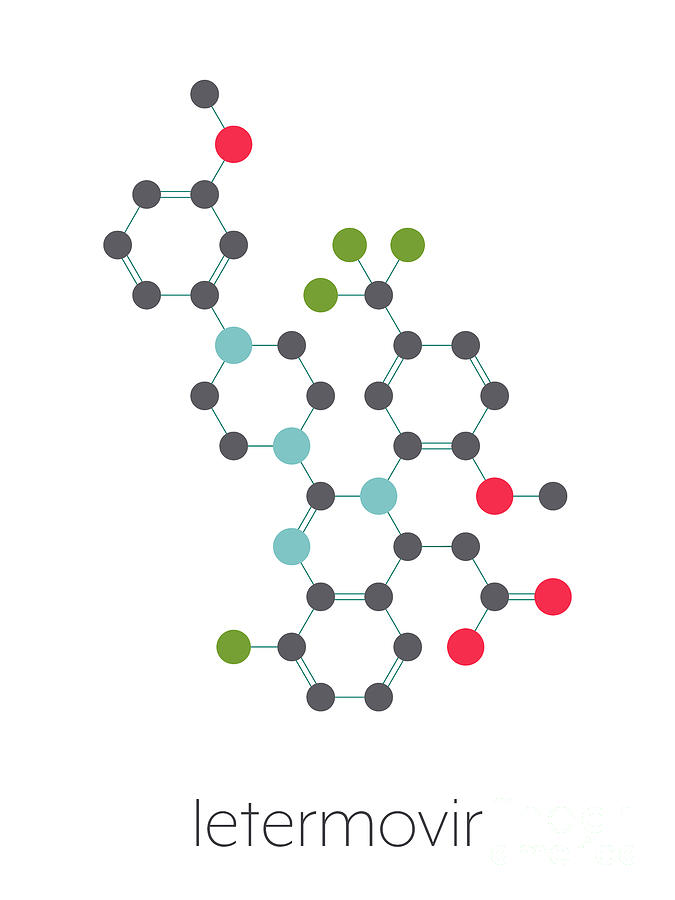


Letermovir Cytomegalovirus Drug Photograph By Molekuul Science Photo Library



Full Text Letermovir And Inhibitors Of The Terminase Complex A Promising New Cl Idr


Letermovir Aic 246 New Drug Approvals



Letermovir Prophylaxis For Cytomegalovirus In Hematopoietic Cell Transplantation Nejm



Letermovir Cytomegalovirus Drug Molecular Model Stock Image F025 2372 Science Photo Library



Letermovir Drug Review


Introduction Clinical Review Report Letermovir Prevymis Ncbi Bookshelf



Maribavir Brincidofovir And Letermovir Efficacy And Safety Of New Antiviral Drugs For Treating Cytomegalovirus Infections Sciencedirect
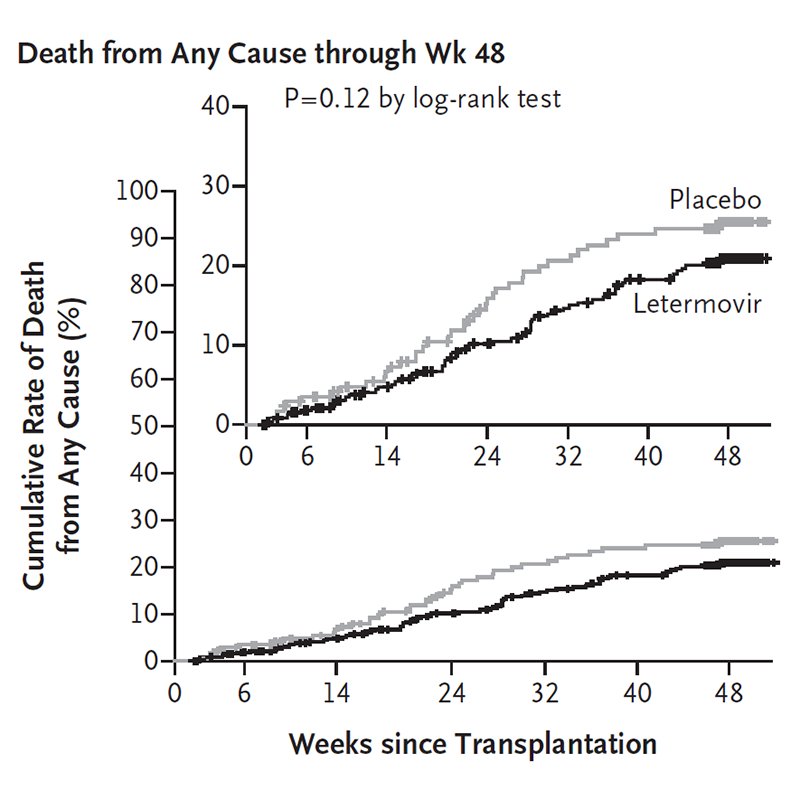


Nejm Original Article Letermovir Prophylaxis For Cytomegalovirus In Hematopoietic Cell Transplantation T Co Urjkudqpim T Co Smqiyrexjl
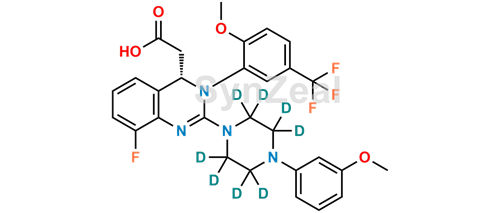


Letermovir D8 9173 32 3 Unlabelled Synzeal



Letermovir



Letermovir Prevymis Uses Dose Side Effects Moa Brands


Q Tbn And9gctt8bmzu2mrgn4yozmskwpnkpzhxk3itg1iteesgczaqvyjdn Usqp Cau
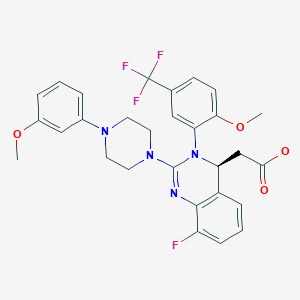


Letermovir Drugs And Lactation Database Lactmed Ncbi Bookshelf



Letermovir For Cmv Prophylaxis In Hsct



Full Text Letermovir For The Prevention Of Cytomegalovirus Infection And Disease Idr


Www Nice Org Uk Guidance Ta591 Documents 1


Letermovir D8



Letermovir 331



These Highlights Do Not Include All The Information Needed To Use Prevymis Safely And Effectively See Full Prescribing Information For Prevymis Prevymis Letermovir Tablets For Oral Use Prevymis Letermovir Injection For Intravenous
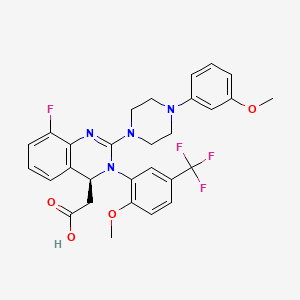


Letermovir C29h28f4n4o4 Pubchem



Letermovir For Cytomegalovirus البورد العراقي للصيدلة السريرية Facebook
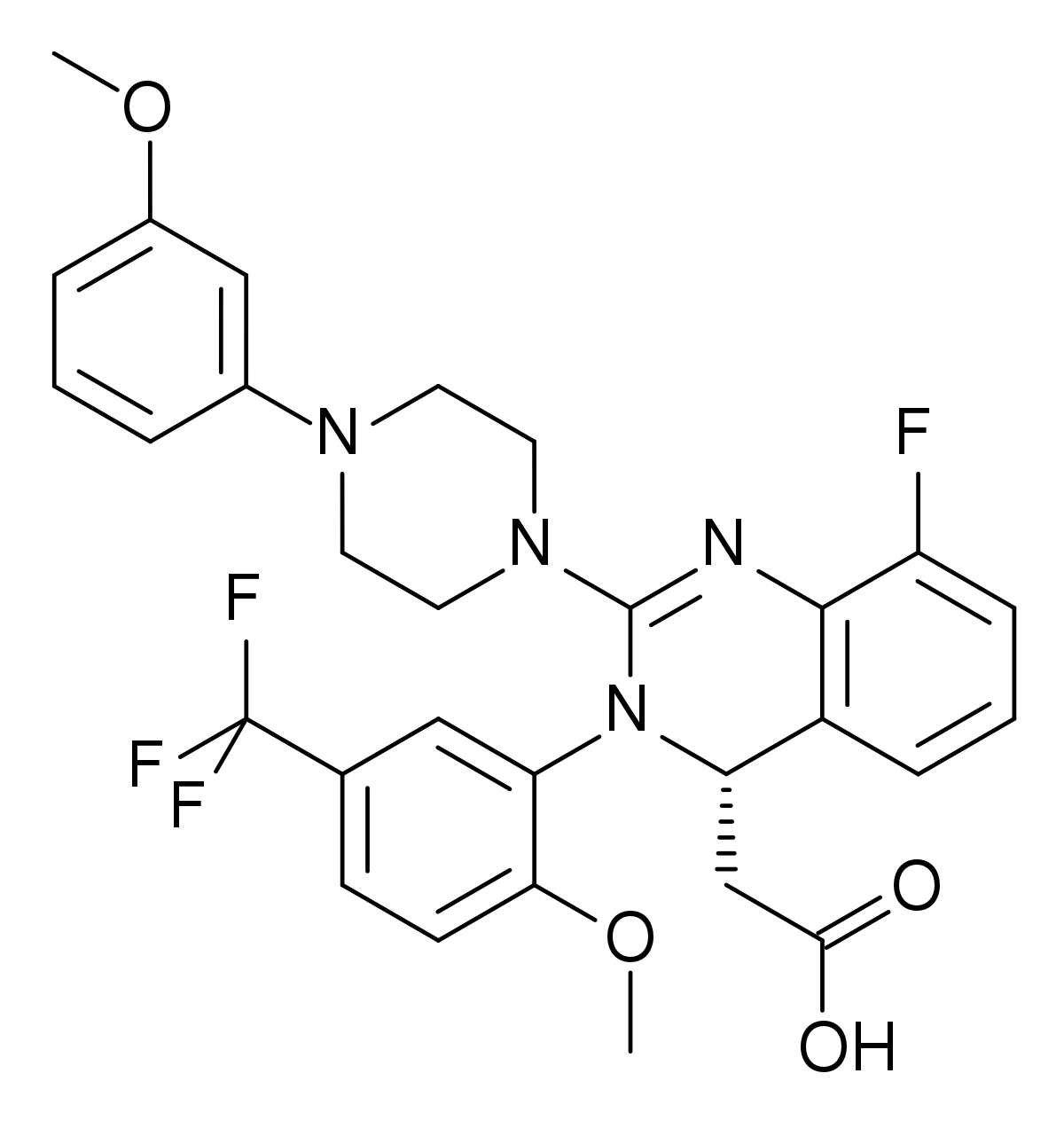


Letermovir Wikipedia
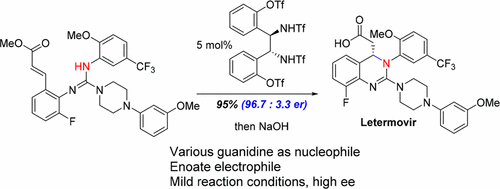


Asymmetric Hydrogen Bonding Catalysis For The Synthesis Of Dihydroquinazoline Containing Antiviral Letermovir Journal Of The American Chemical Society X Mol
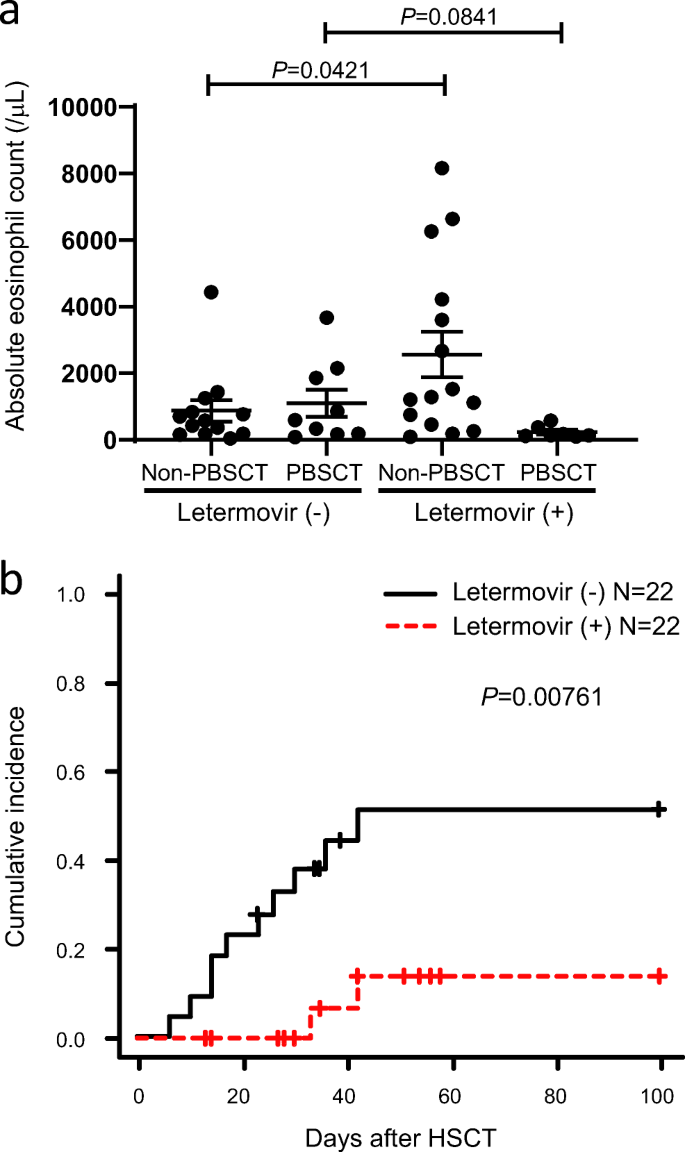


Eosinophilia During Letermovir Treatment After Allogeneic Hematopoietic Stem Cell Transplantation Springerlink
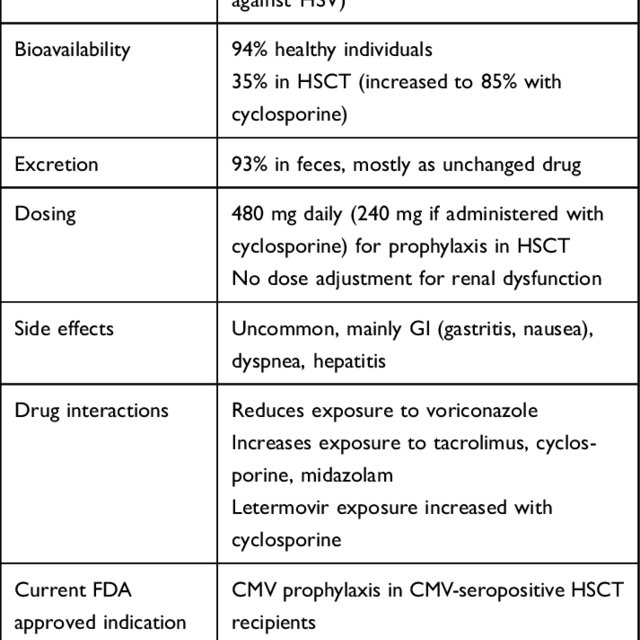


Letermovir Key Characteristics Download Scientific Diagram


Academic Oup Com Cid Advance Article Pdf Doi 10 1093 Cid Ciaa1713 Ciaa1713 Pdf


Www Tandfonline Com Doi Pdf 10 1080 18



Clinical Development Of Letermovir And Maribavir Overview Of Human Cytomegalovirus Drug Resistance Sciencedirect


Http Forumresearch Org Storage Documents Cmv Cmv forum 4 Industry updates Merck Pdf


Letermovir Cytomegalovirus Cmv Drug Molecule Stock Illustration Illustration Of Pharma Pul56
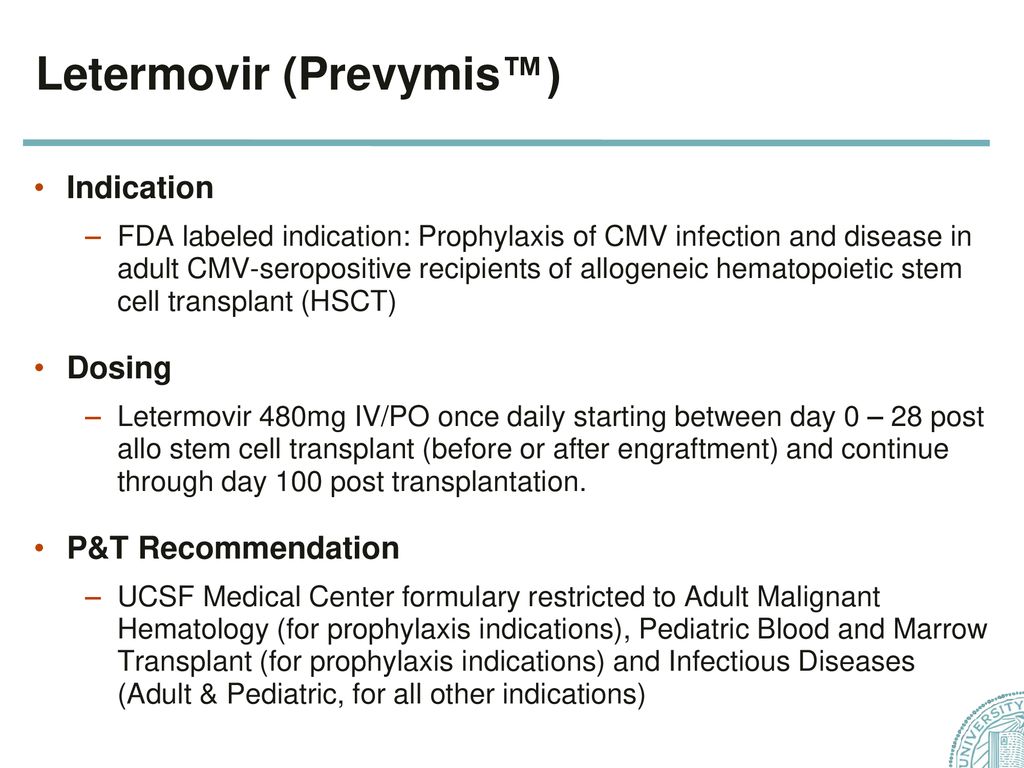


Letermovir Prevymis Guidelines For Inpatient Use Ppt Download



Phase 3 Mortality Analysis In Clinical Trials Prevymis Letermovir
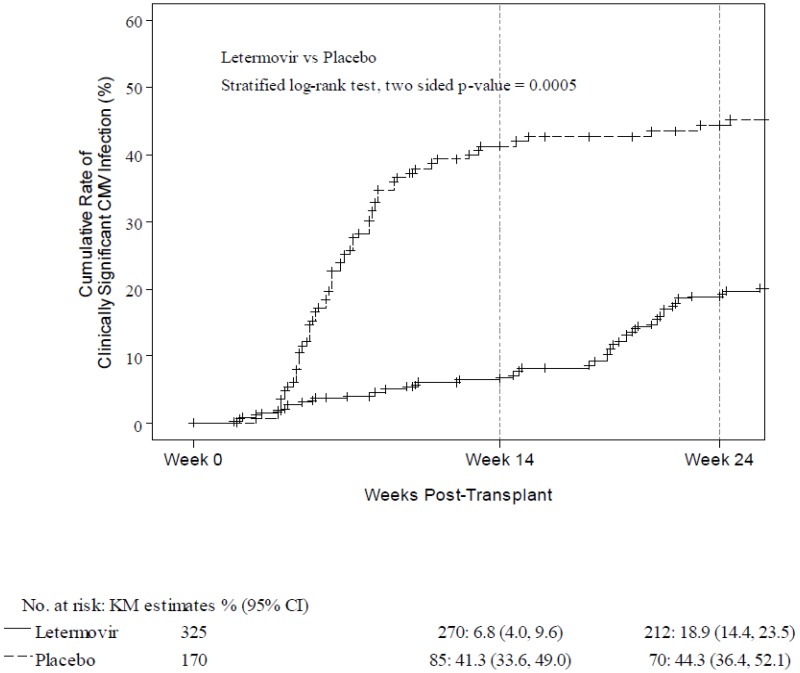


Detailed Outcome Data Clinical Review Report Letermovir Prevymis Ncbi Bookshelf



Prevymis Letermovir Tablets Uses Dosage Side Effects Interactions Warning


Www Tandfonline Com Doi Pdf 10 1080 18


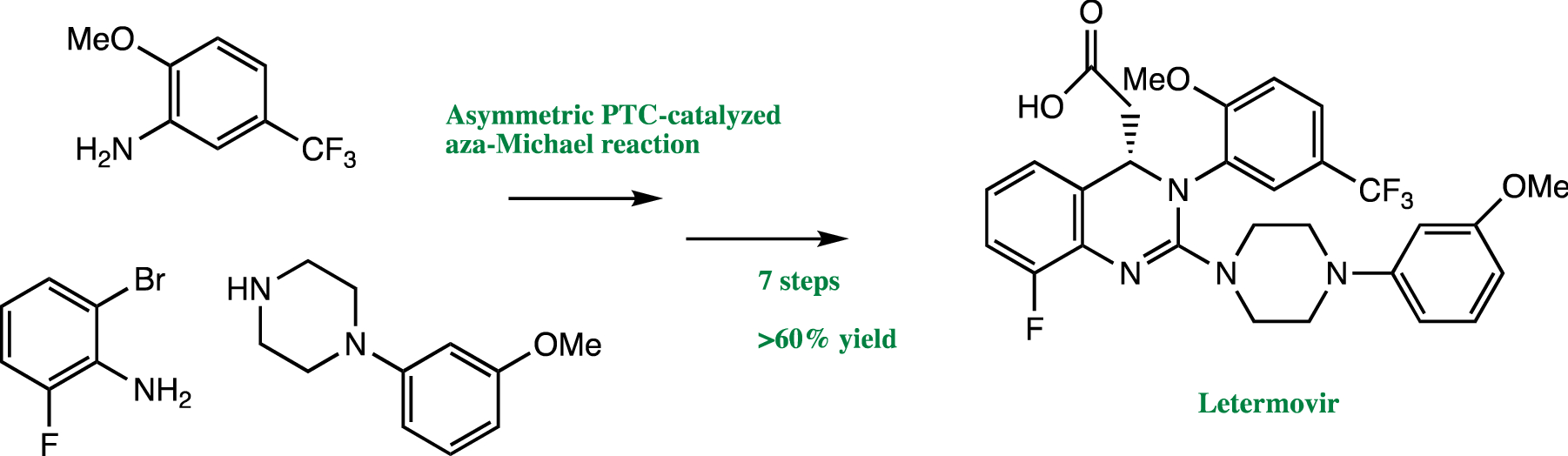
Combining Traditional 2d And Modern Physical Organic Derived Descriptors To Predict Enhanced Enantioselectivity For The Key Aza Michael Conjugate Addition In The Synthesis Of Prevymis Letermovir Chemical Science Rsc Publishing


Pdf Letermovir For The Compassionate Therapeutic Use Of Cytomegalovirus Infection


Letermovir



Letermovir For Secondary Prophylaxis Of Cytomegalovirus Infection And Disease After Allogeneic Hematopoietic Cell Transplantation Results From The French Compassionate Program Biology Of Blood And Marrow Transplantation



China Letermovir Intermediates China 9173 21 0 Letermovir Intermediates



Letermovir Mk Cas 9173 32 3 Antiviral Medkoo
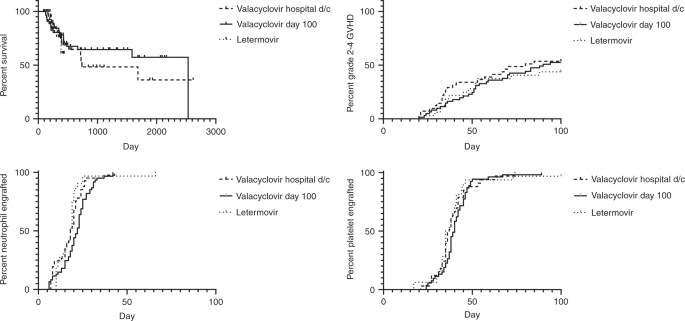


Letermovir Prophylaxis Through Day 100 Post Transplant Is Safe And Effective Compared With Alternative Cmv Prophylaxis Strategies Following Adult Cord Blood And Haploidentical Cord Blood Transplantation Bone Marrow Transplantation


Www Accessdata Fda Gov Drugsatfda Docs Nda 17 9939orig1s000 9940orig1s000medr Pdf
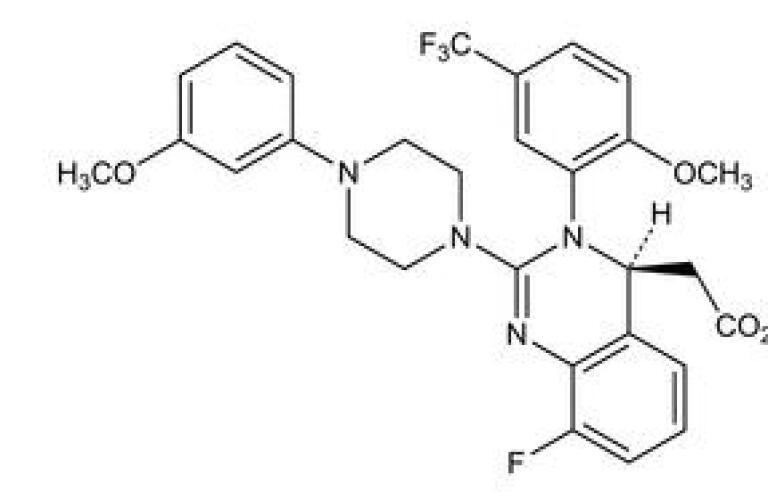


Prevymis Pharmacological Profile Healthgrades Letermovir Injection Solution
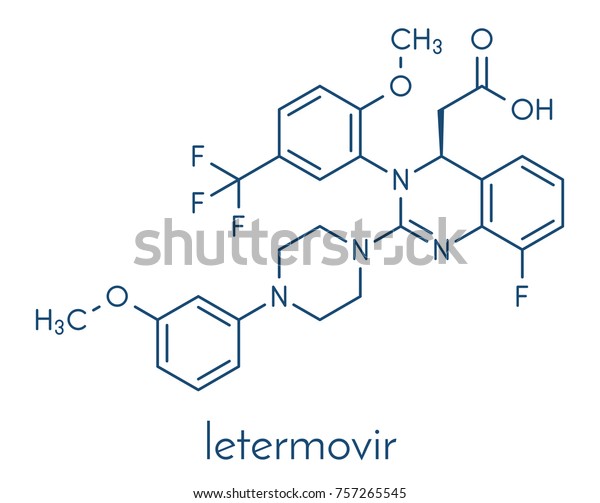


Letermovir Cytomegalovirus Cmv Drug Molecule Skeletal Stock Vector Royalty Free
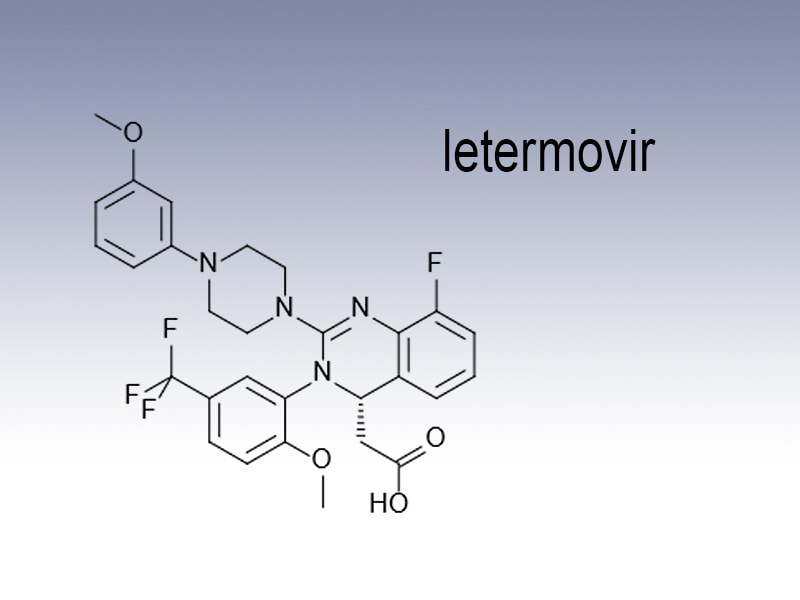


Letermovir Prevymis Okayed For Stem Cell Transplant Infection



Letermovir Racemic Mixture 51 3 Reference Standards Alsachim



Letermovir Targetmol


コメント
コメントを投稿